Abstract
Introduction In 2013, we founded a hematology care network consisting of outpatient centers and the hematological department of the Hanusch Krankenhaus (hospital) within one Austrian health care provider called the Austrian Health Insurance Fund. Our entire medical staff is working within this network. This allows consistent standards from diagnosis to treatment. Additionally, consistent surveillance and close observation after treatment are guaranteed within our network. This includes diagnosis and treatment of coexisting conditions in the outpatient setting. If disease progression is observed, retreatment is performed at the hemato-oncological center at the Hanusch Krankenhaus. Patient fluctuation is low. Therefore, we think that data obtained from our patient population represent real world evidence.
Previously published data suggest that about 60% of myeloma patients proceed to second-line treatment (Fonseca et al., 2020). Here we investigate how many from our patients suffering from multiple myeloma proceed to second-line treatment. We also assessed mortality from first- to second-line treatment.
Methods This retrospective study was conducted in our hematology care network. We included patients suffering from multiple myeloma who received first-line treatment within our network between 2012 and 2021. Follow-up was carried out until July 2022. We were interested in assessing how many patients proceed from first- to second-line, thus patients lost to follow-up and patients under active first-line treatment were not included in analysis. For overall survival (OS), all patients were included, because survival data can be obtained from a central register.
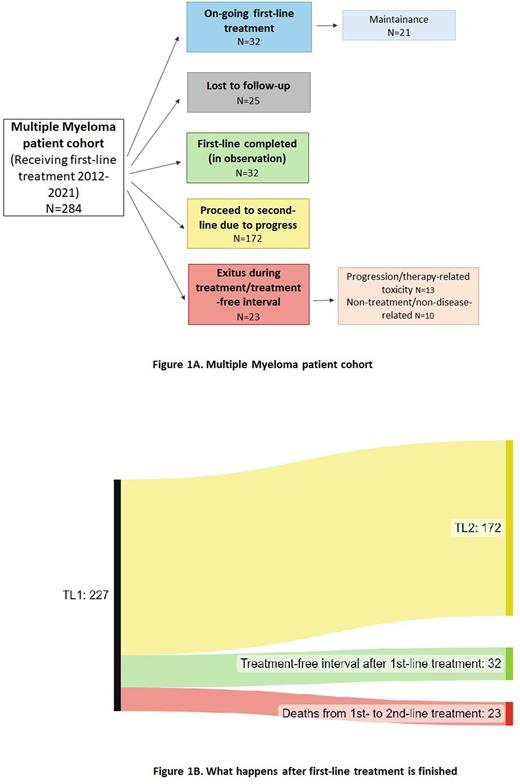
Results Overall, 284 patients received a first-line treatment within our hematological network (Figure 1A). Out of these, 32 patients are under first-line treatment so far without a sign of progression, with 21 patients currently receiving maintenance therapy. Twenty-five patients were lost to follow-up, of these 12 patients were transferred to our institution for ASCT, further treatment and/or observation was performed at the referring sites. Another 13 patients left our institution after first-line treatment. Thirty-two patients completed first-line therapy and are showing no signs of progression, these patients are under surveillance and are eligible for a future second-line treatment. During the observation period, 172 patients proceeded to second-line treatment due to disease progression. Finally, twenty-three patients died during first-line treatment or the subsequent treatment-free interval so far. Out of these, 13 patients died because of disease progression or therapy-related toxicity. The other 10 patients died of causes unrelated to disease or treatment. Two-hundred-twenty-seven patients finished first-line treatment at our institution and are/were in the surveillance program within our network after first-line treatment. Out of these, 172 patients proceeded to second-line treatment, 32 are in a treatment-free interval so far and 23 patients died from first- to second-line treatment (Figure 1B).
Patients receiving treatment containing ASCT (N = 131) had a median OS of 11.6 years. This compared to a median OS of 5.3 years for patients receiving a triple/quadruple therapy regimen without ASCT (N = 65) and a median OS of 3.3 years for more unfit patients receiving a two-drug regimen (N = 84). According to comorbidities, impaired kidney function at any time during observation was a strong predictor of survival. Patients with a creatinine clearance (CrCl) of ≥60 ml/min during follow-up (N=81) showed a median OS of 15.4 years. Patients with at least one CrCl <60 ml/min during follow-up (N=196) showed a significantly lower median OS of 5.2 years.
Conclusion
Of our patients, 75.7% already received a second-line treatment, and mortality rate was low. Only 23 patients died during or after first-line treatment and even when calculating unknown reasons of death as disease-related, mortality rate does not exceed 10%. Additionally, it is likely that patients who are under surveillance and without any sign of disease after first-line treatment will be able to receive a second-line treatment. Thus, we conclude that age and age-related disease rather than myeloma-related factors are responsible for a drop-out from first- to second-line treatment.
Disclosures
Keil:Abbvie: Honoraria, Research Funding; Astra Zeneca: Honoraria; BMS: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Roche: Honoraria; Janssen: Honoraria; Gilead: Honoraria; Takeda: Honoraria, Research Funding. Koller:Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pfeilstöcker:BMS: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal